Tailored Solution for your Compliance Needs
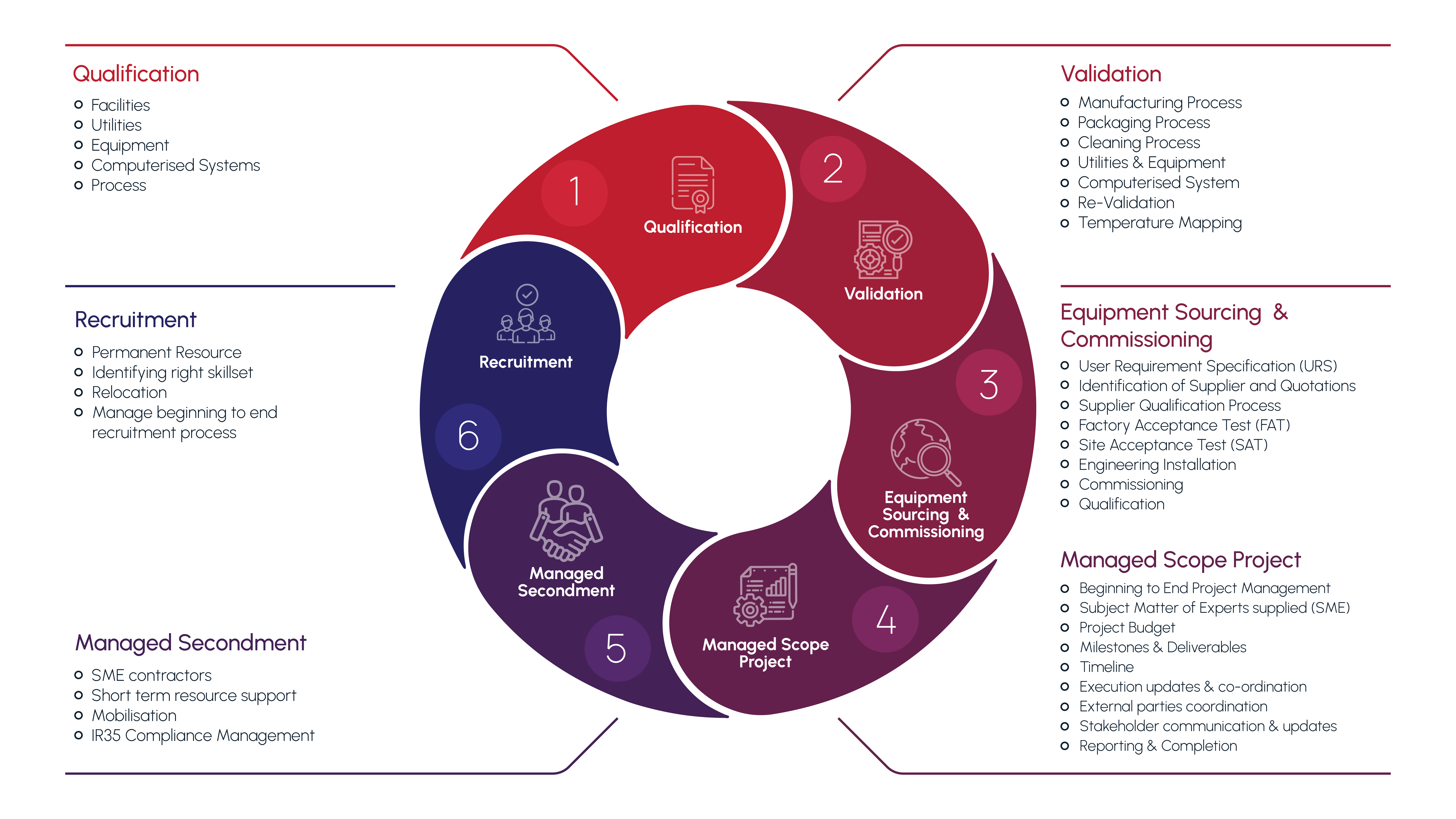
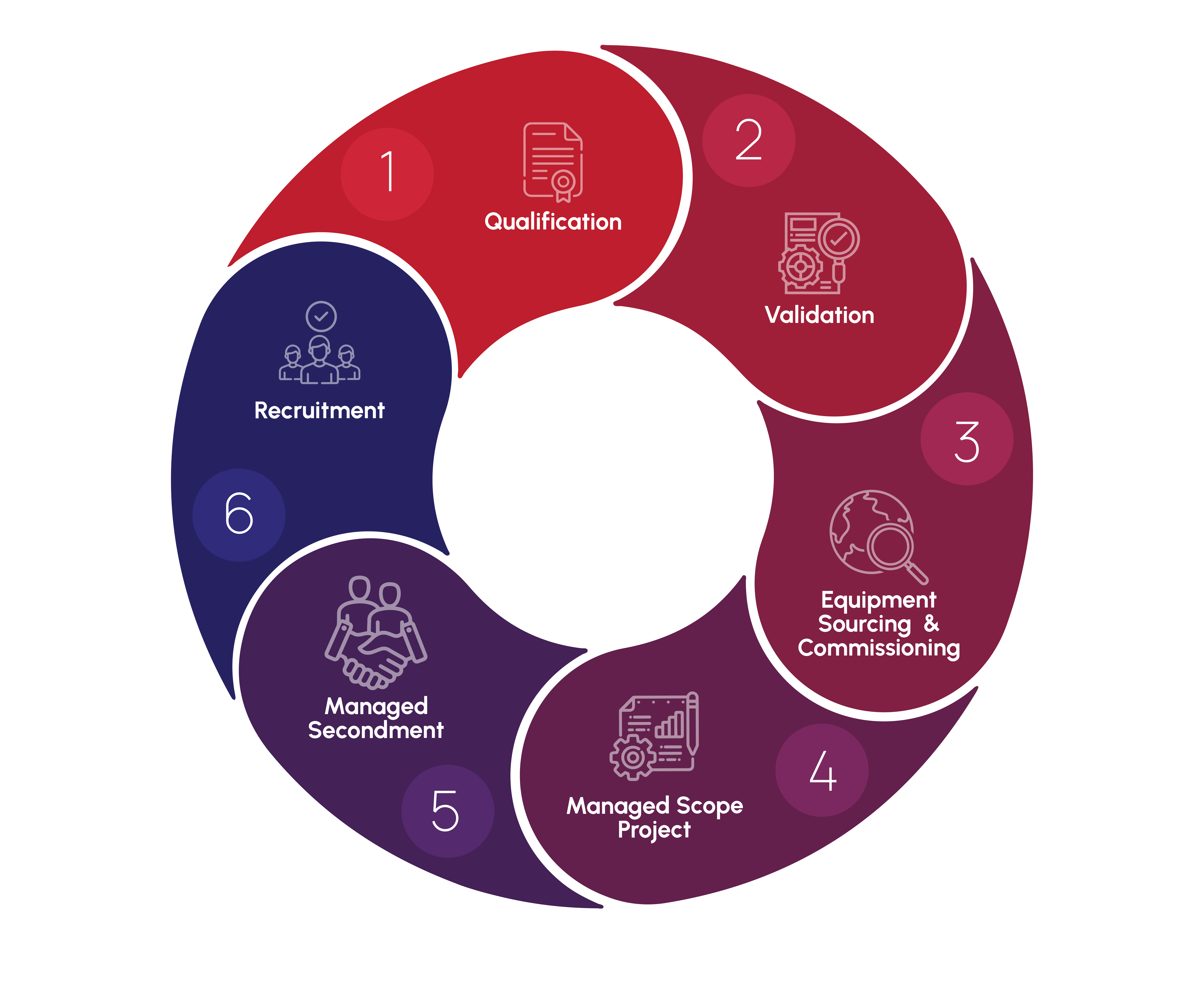
1. Qualification
- Facilities
- Utilities
- Equipment
- Computerised System
- Process
2. Validation
- Manufacturing Process
- Packaging Process
- Cleaning Process
- Utilities & Equipment
- Computerised System
- Re-Validation
- Temperature Mapping
3. Equipment Sourcing & commissioning
- User Requirement Specification (URS)
- Identification of Supplier and Quotations
- Supplier Qualification Process
- Factory Acceptance Test (FAT)
- Site Acceptance Test (SAT)
- Engineering Installation
- Commissioning
- Qualification
4. Managed Scope Project
- Beginning to End Project Management
- Subject Matter of Experts supplied (SME)
- Project Budget
- Milestones & Deliverables
- Timeline
- Execution updates & co-ordination
- External parties coordination
- Stakeholder communication & updates
- Reporting & Completion
5. Managed Secondment
- SME contractors
- Short term resource support
- Mobilisation
- IR35 Compliance Management
6. Recruitment
- Permanent Resource
- Identifying right skillset
- Relocation
- Manage beginning to and recruitment process
Supported by our valued
partners around the globe
Empowering Excellence in
Pharmaceutical Solutions
At the heart of our mission lies a commitment to delivering exceptional commissioning, qualification, and validation (CQV) services to traditional and biotech pharmaceutical clients. With a foundation built on experience, passion, and unwavering quality.
We strive to provide best-in-class solutions that meet the unique needs of our clients. Our vision is to become the most respected, passionate, and trusted CQV consultancy across the UK and Europe, setting new standards of excellence in the industry.