Service Details
- Home
- Service Details
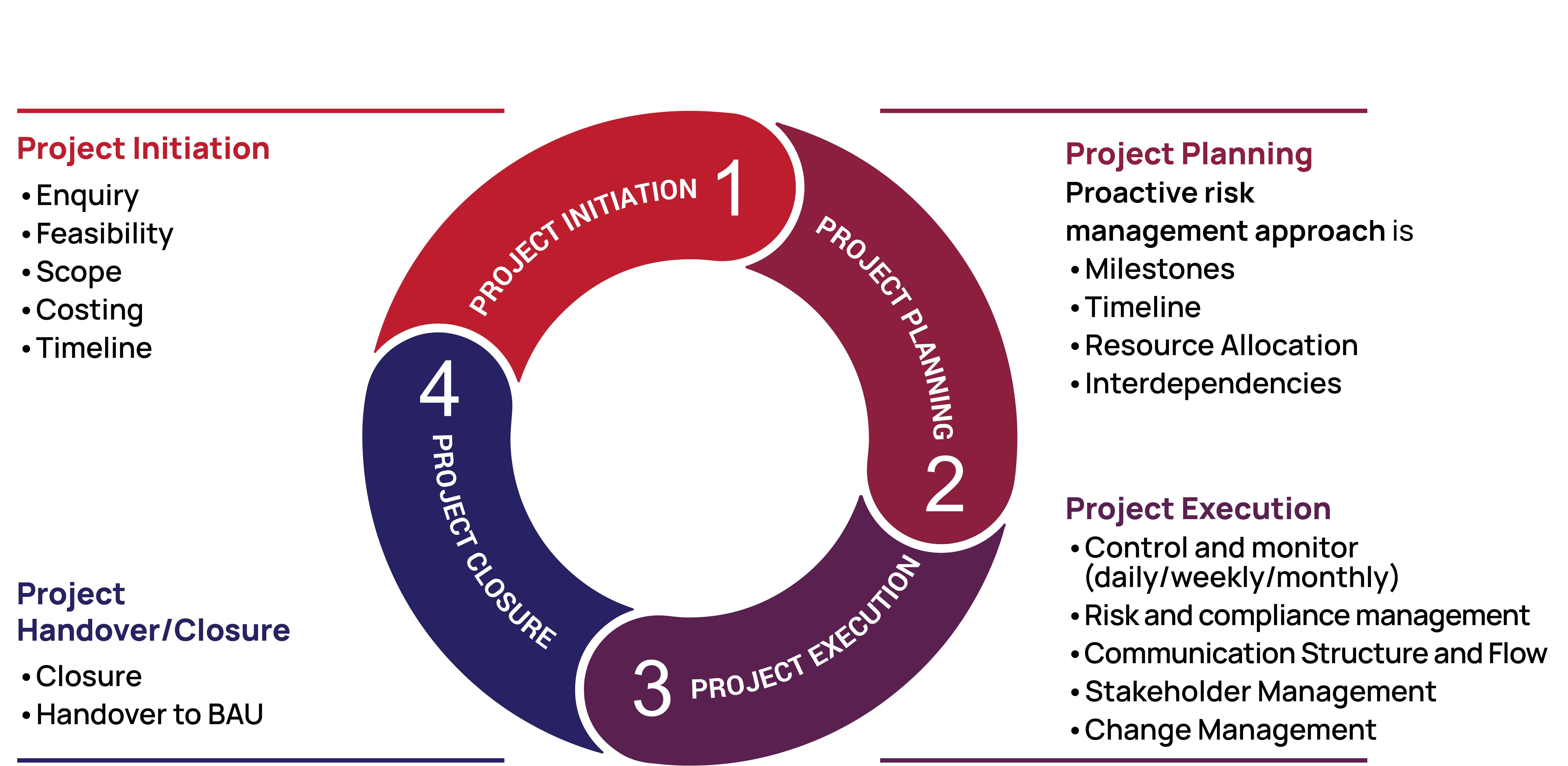
Managed Scope Project
Navigating the ever-evolving regulatory landscape of the biotech, pharmaceutical and medical device industries can be challenging. Our CQV compliance experts possess years of combined experience, ensuring your project is managed effectively and validation programmes are executed flawlessly. Our primary objective is to ensure your inspections meet the highest quality and compliance standards.
• Risk-Based Approach
• Efficient Project Management
• Effective Communication
• Collaboration & Coordination
• Experience in providing Effective Solutions
• Stakeholder Management
• Quality & Compliance
• Agile & Streamlined Approach
• Subject Matter of Experts in Multiple Areas